Tuesday January 12, 2020 | VANCOUVER ISLAND, BC [Updated 8 pm]
by Kiley Verbowski | Island Social Trends | Mary P Brooke, B.Sc., editor
As of Monday January 11 a total of 59,902 British Columbians have received the COVID-19 vaccine and health care workers are continuing to poke vaccines into the arms of the province’s most vulnerable populations.
In a joint statement this week, Provincial Health Officer Dr. Bonnie Henry and Health Minister Adrian Dix said that “our focus is to ensure we safely deliver the vaccines as quickly as possible to communities throughout the province, using all available supply.”
To date, the vaccines administered to frontline workers in long-term care and health-care have been mostly the Pfizer-BioNTech product, while Indigenous people in rural and remote communities received the Moderna vaccine due to easier shipping requirements. Both products are the mRNA type. Both products require a second (booster) shot which Dr Henry says will come around 35 days after the first shot.
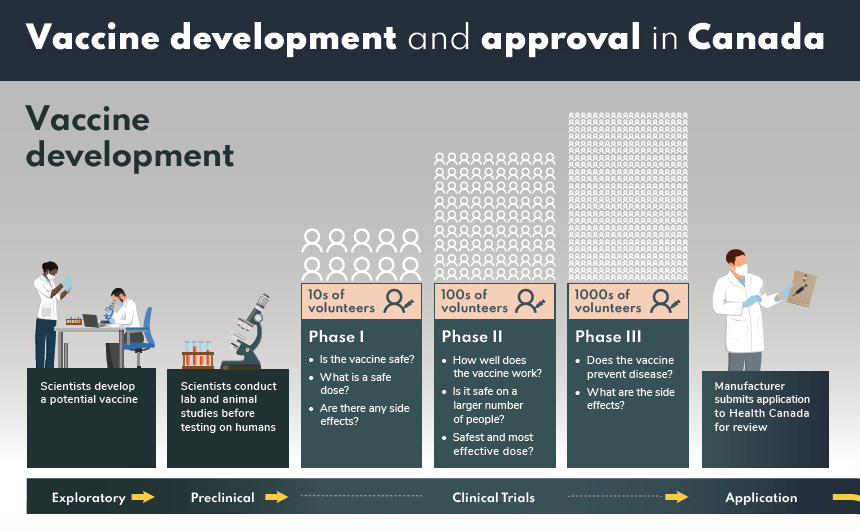
The decision about prioritizing as to who should be immunized first was made last month, which will account for all dosages as known to be presently available up until the end of March, though today that number changed again as the federal government announced the purchase of another 20 million Pfizer-BioNTech vaccine doses to come in this first quarter of 2021.
As to which age demographics or industry sectors receive vaccines next, is information yet to come, said Dr Henry on Monday.
Scope of the problem:
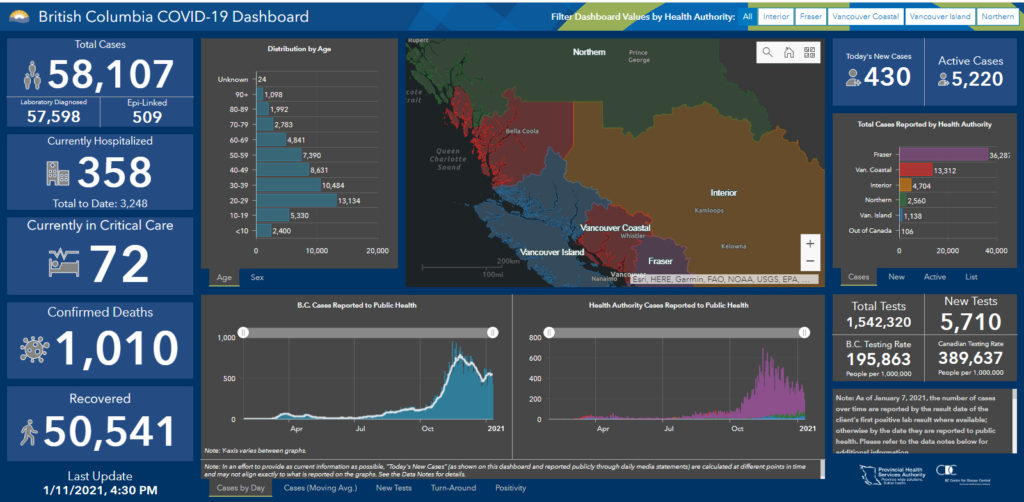
Note that to date (January 11), there have been 58,107 cases of COVID-19 in BC of which 1,138 have been on Vancouver Island. Among children under the age of 10, there have been 2,400 cases in BC during the pandemic to date, with 60 of those on Vancouver Island; among youth ages 10 to 19 there have been 5,330 cases to date, with 107 of those on Vancouver Island. There have been 1,010 deaths in BC to date due to COVID-19 (13 in Island Health).
Currently there are 5,220 active cases in BC (mostly in the lower mainland area), with 175 currently active on Vancouver Island. There are 358 hospitalizations for COVID-19 in BC, with 72 in ICU; on Vancouver Island eight people are hospitalized with one in ICU.
Socioeconomically there is a wide range of impact due to COVID-19, affecting the social, economic, career and mental health aspects of nearly every person in some way in 2020 and going forward. A lower income bracket adds more challenges to the availability of coping strategies. Governments at all levels are using various strategies to help people cope and adapt.
Who’s been vaccinated and when will you get the vaccine?:
The first group of people (approximately 150,000) to be vaccinated between mid-December 2020 and February 2021 are listed by BC Health as:
- residents, staff and essential visitors to long-term care and assisted-living residences;
- individuals in hospital or community awaiting a long term care placement;
- health care workers providing care for COVID-19 patients in settings like Intensive Care Units, emergency departments, medical/surgical units and paramedics;
- remote and isolated First Nations communities.
The second group (approximately 400,000), to be vaccinated between February and March are:
- Community-based seniors, age 80 and above
- Indigenous (First Nations, Métis and Inuit) seniors, age 65 and above
- Indigenous elders
- People experiencing homelessness and/or using shelters
- Provincial correctional facilities
- Adults in group homes or mental health residential care
- Long-term care home support recipients and staff
- Hospital staff, community GPs and medical specialists
- Other Indigenous communities not vaccinated in first priority group
The provincial government says that “additional groups may be prioritized for vaccination” as transmission continues to be monitored. The next groups are expected to be announced in the next few weeks.
Some experiences up close:
Boston Laferté is a 20-year-old studying History and Indigenous Studies at the University of Victoria. He will most likely be in one of the last groups to receive the vaccine considering his age and healthy immune system. “I get that there are restraints on vaccine supplies and processes,” he says, and understands why young, healthy people may have to wait longer.
For young people working high-risk jobs, like Sophie Underwood who is a 24-year-old childcare worker in the Victoria area, the government’s priorities and messaging has been confusing.
“I have seen little messaging for someone like me that doesn’t live alone or with my family,” she said. “There has been no guidance about how to talk to roommates about the guidelines, or how to navigate people in my bubble returning from travel.”
She says her job is essential as long as schools remain open, and would like to be vaccinated at the same time that teachers are, although she has doubts about that happening. If the vaccine rolls out by decreasing age bracket “and a 40-year-old working from home gets vaccinated before a 20-year-old working a high contact job, well that feels pretty bad.”
What you need to know about the Pfizer-BioNTech vaccine:
- It was the first SARS-CoV-2 vaccine to be approved for use in Canada. Health Canada gave it the go-ahead on December 9, 2020.
- Approved for people age 16 years and older.
- Requires two doses (0.3mL), 21 days apart. [Note: in BC that will be up to 35 days apart.]
- Doses of the vaccine must be stored and transported in an ultra cold freezer between -60˚C and -80˚C, and may take up to two hours to thaw at room temperature before being administered and then must be used right away.
- Vials contain six doses, and must be diluted with saline diluent.
- It is administered by a needle in the deltoid muscle (upper arm).
- It is 95 per cent effective in preventing COVID-19 beginning one week after the second dose, based on a study with about 44,000 participants.
- Recipients of the vaccine will not be fully protected from the disease until at least seven days after the second dose. In total, immunization takes 28 days according to manufacturer’s specs (stretched to 35 in BC based on study of the manufacturer’s data; the World Health Organization says up to 42 days is okay).
- A complete ingredient list can be found here.
What you need to know about the Moderna vaccine:
- Approved in Canada on December 23, 2020, Moderna is the second vaccine to have been approved to combat COVID-19 (SARS-CoV-2)
- It is approved for use in people age 18 and older.
- It requires two doses (0.5mL), 28 days apart. [In BC that will be 35 days.]
- Doses of the vaccine must be stored and transported at a temperature between -15C and -25C, and take one hour to thaw at room temperature. For reference, a household freezer is usually -18C.
- Vials contain 10 doses. The product does not need to be diluted.
- It is administered by a needle in the deltoid muscle (upper arm).
- It is 94.1 per cent effective in preventing COVID-19 beginning two weeks after the second dose.
- Recipients of the vaccine will not be fully protected from the disease for at least 14 days after the second dose. In total, immunization takes 42 days.
- A complete ingredient list can be found here.
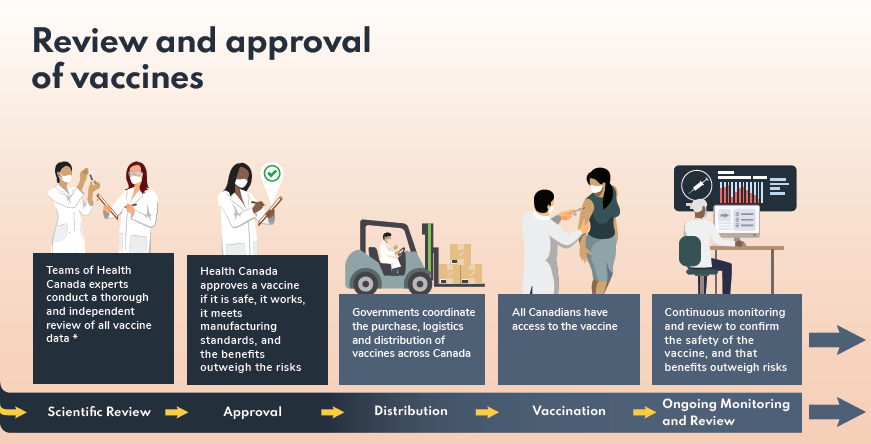
What does mRNA mean?:
Messenger RNA is a fairly new type of technology used in the Pfizer-BioNTech and Moderna vaccines. Instead of putting a weakened or inactive version of the virus into our bodies, the new vaccines teach cells how to make a protein or piece of protein that triggers an immune response inside the human body. The body will remember and recreate that protein if it comes in contact with the SARS-CoV-2 coronavirus.
Proteins are incredibly hard-working parts of the human body. They form the structure of cells, make tissues, fuel chemical reactions, and send messages. They are essential to all of the body’s functions. Starting in 2005, scientists could manipulate these processes in humans, and in 2020 got the opportunity to quickly develop and test an mRNA vaccine for COVID-19.
Scientists say that long-term trials need to be conducted before definitively saying that mRNA vaccines are better than other options, but they do have some clear advantages. One is that typical vaccines are created using egg protein, which can prompt an allergic reaction in some people.
More vaccines are on the horizon:
A vaccine by Johnson & Johnson and Janssen is currently in Phase 3 of clinical trials, with around 45,000 participants. Unlike the two vaccines already approved by Health Canada, this vaccine only requires one dose, and can be kept at refrigeration temperatures. If trials continue to be successful, the vaccine may be approved before the end of January.
A vaccine by AstraZeneca and Oxford University has recently been approved in the UK and Australia. It can be stored for longer periods at normal refrigeration temperatures. Its efficacy has had differing results with different doses: only 62 per cent for participants given two full doses, but 90 per cent for a group that was given a half, then a full dose a month later. A timeline on possible approval by Health Canada has not been provided.
U.S. company Novavax is running trials in Britain, and although it has not submitted an application to Health Canada yet, the federal government has already signed up for 76 million doses upon approval. It requires two doses, 21 days apart. This vaccine differs in that it contains a lab-made version of the SARS-CoV-2 spike protein, and will be mixed with an adjuvant (an agent commonly added to vaccines to boost the body’s immune response).

A Quebec-based company called Medicago is currently in Phase 1 trials, and the federal government has invested up to $173 million into the company’s research and production, and signed on for 76 million doses upon approval by Health Canada. The unique vaccine is created by infecting plants with genetically modified bacteria that produces several SARS-CoV-2 proteins. Virus-like particles are then extracted from the leaves and purified.
A vaccine made by joint French and British companies Sanofi and GlaxoSmithKline announced a set back during mid-stage trials, and say they will start a new study in February 2021. Canada has an agreement for 72 million doses upon approval.
Testing on children and teenagers for the Pfizer and Moderna vaccines is already in progress.
Will the vaccine solve everything?
The BC provincial government estimates that everyone in the province that will be vaccinated will have the opportunity to do so before the end of 2021. the federal government is using “by September” as a benchmark for vaccination success across the country (i.e. enough to achieve herd immunity).
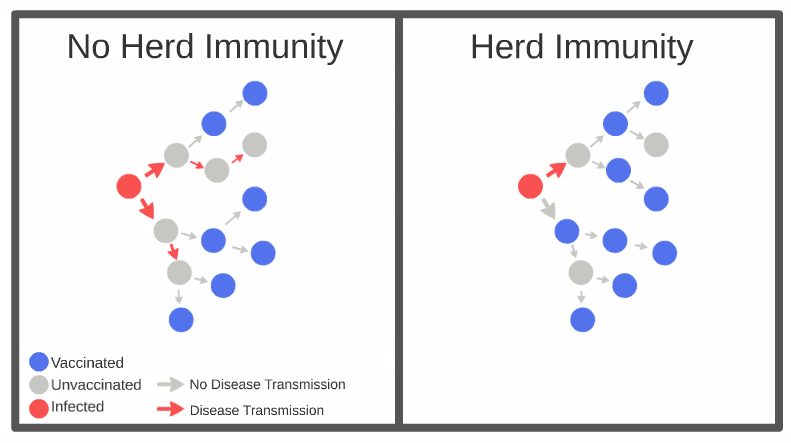
In order to reach herd (community) immunity, 60 to 70 per cent of the population will need to receive the vaccine. That does not ensure that breakouts will cease, but rather that the disease will not be able to spread through the population very rapidly, and most people will not get sick.
Researchers are not sure if being immunized will mean that recipients will not be able to transmit the disease if they come in contact with it. Scientists have noted that this isn’t much of a concern, because having a majority of the population immunized would reduce the rate of transmission by about 95 per cent anyways.
In more good news, the currently approved vaccines will protect against the B.1.1.7 strain of COVID-19 (first dubbed as the UK variant, though public health is trying to adhere to its general policy of not associating geographical names to a virus).

The SARS-CoV-2 virus will continue to mutate, but the current vaccines should continue to protect recipients for many years (perhaps with boosters required, even annually, similar to what is done annually for influenza protection).
If the disease does out-mutate the vaccine, solutions created with mRNA ensure that scientists will not have to start from scratch to create a new vaccine.
Keeping pace:
Presently all vaccine doses that are received in BC are set to use within the priority hierarchy as outlined by public health. The system is trying to keep up with the challenges of both the virus itself and the logistics of distributing and administering it.
Are there any risks involved to getting immunized?:
The COVID-19 vaccines do not contain the live virus, and therefore cannot give the recipient COVID-19.
Common reactions to the vaccine may include soreness, redness and swelling at the site where the vaccine was given. Other reactions include tiredness, headache, fever, chills, muscle or joint soreness, nausea and vomiting. These reactions are mild and generally last one to two days.
Serious side effects of the vaccine were not seen in the clinical trials, which is a main reason that governments and public health have been keen to assure confidence in the vaccine product as approved to date.
Recipients are asked to stay in the clinic for 15 minutes after receiving any vaccine because about one in a million people can experience anaphylaxis. This may include hives, difficulty breathing, or swelling of the throat, tongue or lips. Should this reaction occur, the health care provider is prepared to treat it. Emergency treatment includes administration of epinephrine (adrenaline) and transfer by ambulance to the nearest emergency department.
The World Health Organization estimates that vaccines save two to three million lives worldwide every year.
How much do the vaccines cost?:
The market price for the Pfizer-BioNTech vaccine is $19 per dose, and the Moderna vaccine is priced between $25 and $37 per dose.
However, Canada has guaranteed that vaccines will be free to all Canadians. The federal government has stated that, “we expect to be able to offer free vaccination to every Canadian who wants one”, and of course the provinces and territories will need to line up with that promise (health care is a provincial/territorial responsibility). Prime Minister Justin Trudeau repeated that again this week.
Another attractive feature of the next vaccines in line for approval in Canada, the Johnson & Johnson and Janssen vaccines, are apparently going to be less expensive at about $10 a dose.
Canada also committed $440 million to support low- and middle-income countries for accessing COVID testing, and receiving treatment and vaccines.
Editor’s Note: The circumstances of vaccine supply, research on the number of days between doses, availability and safety for certain population groups (e.g. pregnant women, children), the price of vaccines, and timelines for delivery are changing pretty much daily as the pandemic continues to rage on in 2021.